"Anode" and "cathode" are fundamental terms used in electrochemistry and electronic circuits. These two types of electrodes play important roles in a variety of systems, from simple batteries to advanced technologies.
Let’s examine their differences, positive and negative labels, and how you can easily know which one is negative or positive.
What Is an Electrode?
It's important that you understand the general idea of what an electrode really is before getting into the intricacies of what a cathode or an anode is. In the most basic sense, an electrode is a material that aids in the conduction of electricity, enabling electric current to enter or exit a non-metallic medium, such as an electrolytic cell.
Simply put, an electrode serves as a conductor to make electrical contact with a non-metallic component of the circuit.
What Is an Anode?
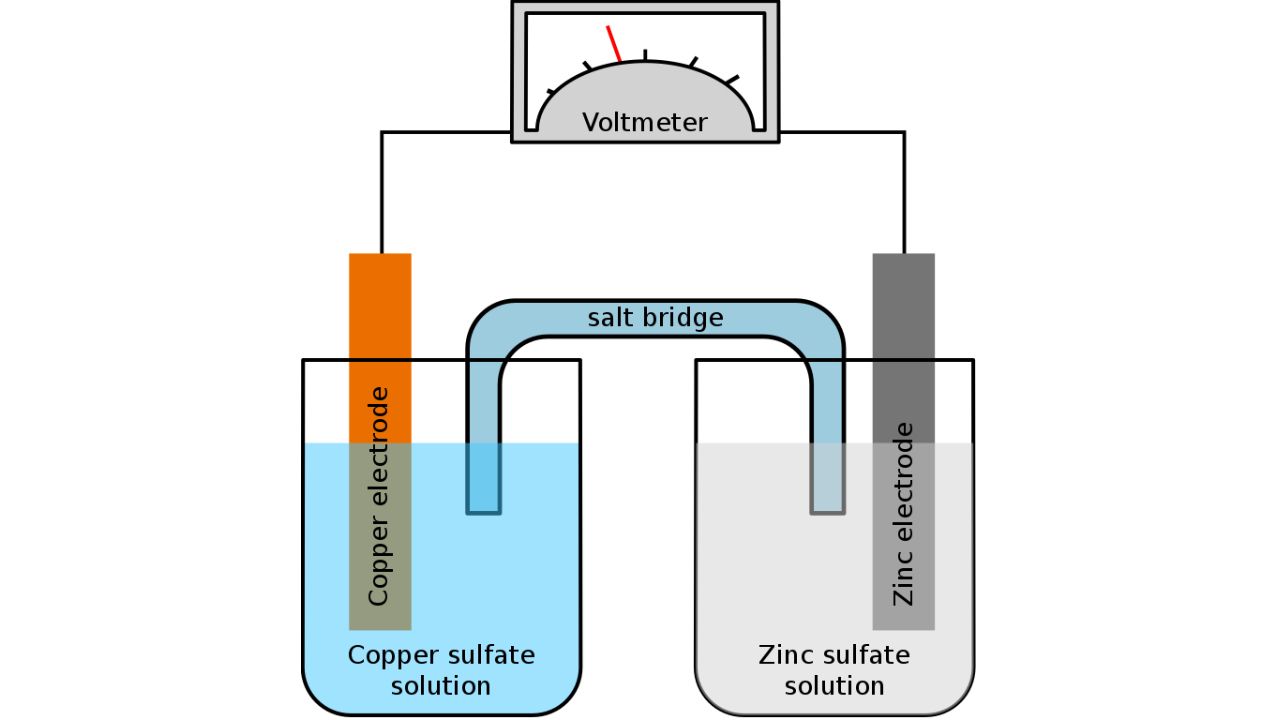
The polarity of an electrode, whether it's an anode or cathode, is dependent on the circuit type. The anode is the electrode where oxidation occurs, resulting in the loss of electrons. Looking at what happens in a galvanic cell (which converts chemical energy into electrical, such as a battery discharging), the anode acts as the negative electrode since, during oxidation, electrons are left behind on the electrode and flow through the external circuit.
Conversely, in electrolysis, where an electric current drives electron flow in the opposite direction, the anode becomes the positive electrode.
What Is a Cathode?
The cathode serves as the site where reduction occurs, facilitating the gain of electrons. In a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode and subsequently plate out.
On the other hand, in electrolysis, the cathode is the negative terminal, attracting positive ions from the solution.
Anode vs. Cathode: Clarifying Polarity
Initially, electricity was believed to flow from positive to negative, the opposite of what we now understand to be true. This early misconception has led some to associate the anode with negativity and the cathode with positivity.
However, with the correct understanding of the roles of oxidation and reduction in each electrode, it becomes easy to identify which one is the anode or cathode—this is dependent on the circuit type.
What Is an Electrolyte in a Battery?
In batteries, the electrolyte refers to the medium that allows the flow of ions between the anode and cathode while keeping them electrically neutral. This ionic movement is essential for the chemical reactions that occur during the charging and discharging processes. To learn more about this, read how lithium-ion EV batteries work.
Which One Is Positive?
To understand polarities when talking about electrodes in a cell or circuit, it’s important to consider the two reactions that take place at the two sites. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis.